Recent study-a-thon investigates the natural history and outcomes of prostate cancer patients managed with watchful waiting. A conservative management option for prostate cancer patients with a life expectancy of less than 10 years at time of diagnosis. The patient’s disease is ‘watched’ for development of local or systemic progression until they require palliative treatment (care that makes a disease or its symptoms less severe or unpleasant but without removing the cause), with the intention being to maintain quality of life.
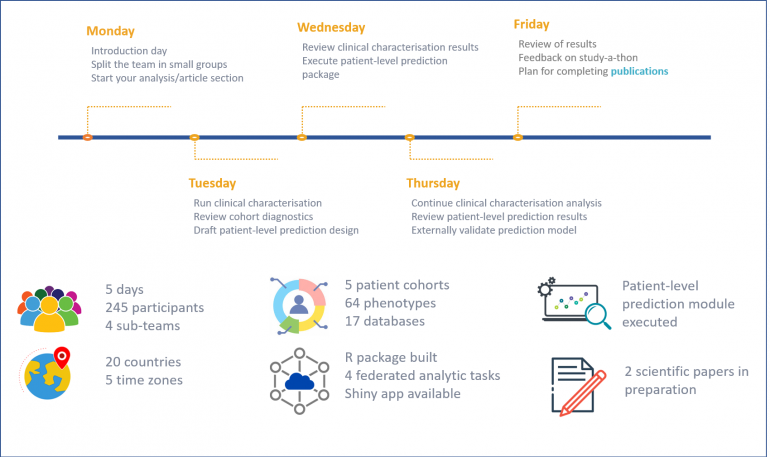
- More than 240 participants including data scientists, clinicians, epidemiologists, patients and statisticians from 20 different countries came together to answer this high priority question.
- 17 databases from across six countries with prostate cancer patient data which had already been mapped to the Observational Medical Outcome Partnership (OMOP) Common data model (CDM) participated, and this number is still growing.
- Two high-impact scientific papers will soon be submitted for peer-review. The first, assessing the impact of comorbidities and life expectancy on the long-term outcomes of patients managed by WW and the second, on the development of prediction models for the time to symptomatic progression, palliative treatment initiation or death within a specific timeframe for watchful waiting patients.
Over 5 days from the 8th-12th of March PIONEER (European Network of Excellence for Big Data in Prostate Cancer) joined forces with EHDEN (European Health Data and Evidence Network) and OHDSI (Observational Health Data Sciences and Informatics community) to host a virtual prostate cancer study-a-thon to inform shared healthcare decision-making for prostate cancer patients managed by watchful waiting. A study-a-thon is a concentration of researchers, epidemiologists, statisticians, clinicians and patients to research a specific clinical question in for instance five days, truncating what would normally take months, or even years.
The aim of this study-a-thon was to assess selection criteria and long-term outcomes of prostate cancer patients on watchful waiting by using an international network of real-world data spanning the years watchful waiting has been a recognised prostate cancer management approach.
Building on the experience of the OHDSI COVID-19 study-a-thon in 2020 and prior EHDEN study-a-thons the PIONEER study-a-thon began on the 8th of March; however, preparation for the event began months beforehand with the:
- Completion of a literature review of 14,996 articles. The aim of the review was to inform
the study regarding the varying definitions of watchful waiting, the outcomes reported
across the studies as well as the characteristics of patients included in the studies.
This information supported the development of the study protocol and informed the
discussion on defining the patient cohorts. - Drafting of the study protocol which guided the Data Sources team and helped them to
identify and invite data holders both internally and externally to PIONEER to partake in
the study; ultimately, making the analytics possible. - Patient outreach initiative. The study-a-thon management team felt very strongly that the
patient voice needed to be represented within the workstreams. To achieve this a number
of patient representatives were invited to share their personal stories of prostate
cancer diagnosis and treatment with the participants as well as to participate in the
Patient Characterisation and Phenotyping workstreams. - Development of a Microsoft Teams environment that acted as the virtual hub for the study-
a-thon. Over the course of the 5 days there were 10 global overarching sessions, 15 team
meetings within the 4 active workstreams with all participants generating over 870
engagements including posts, replies, reactions and mentions across the platform.
The study-a-thon participants were divided across four workstreams/sub-teams.
Clinical characterisation who described the demographic and clinical characteristics of patients with prostate cancer (PCa) under conservative management & estimated clinical outcomes of these patients including those who initiated treatment. Cohort diagnostics and clinical characterisation results from several databases available covering 340,000 unique patients. Explore the clinical characterisation results on Shiny App.
Phenotyping who defined the study phenotypes/cohorts clearly, unambiguously and accurately to generate meaningfully evidence considering differences/nuances of the databases. To date 64 phenotypes have been defined via the ATLAS tool.
The Prediction team who developed a prediction model, in the context of WW, that predicts an outcome (symptomatic progression, death, death without symptoms) at a specific moment in time (6, 12, 24 months) based on a combination of patient characteristics.
Last, but perhaps the most integral team Data Sources and Study Execution who identified and recruited appropriate databases to the study; developed the code to run analyses for clinical characterisation and to compile results in an easy-to-install R package; and debugged and adapted the code as required to match the requirements of the different data providers and to reflect changes in the research question.
One of the highlights of the study-a-thon was the inclusion of patient representatives. Over the course of the 5 days four prostate cancer patients shared their personal stories of their prostate cancer diagnosis, treatment, and ongoing follow-up where applicable. The patients also made valuable contributes to the clinical characterisation and phenotyping teams by bring their unique perspective to the discussions. Overall, participants found that including patients in the study helped them gain additional perspectives on their work and impacted greatly with regards to framing how the study-a-thon outcomes should be used and disseminated to different stakeholder groups.
Over the course of the next month the study-a-thon team will continue to run analysis across more databases adding to the results of the clinical characterisation study, whilst the prediction team will work towards external validation of their prediction model. It is hoped this continued collaboration, with weekly team meetings, will culminate in the submission of two high-impact scientific papers (both already drafted) and an exciting presentation of the PIONEER study-a-thon team to the OHDSI community in May. For updates on the PIONEER study-a-thon or PIONEER in general follow us on Twitter @ProstatePIONEER or LinkedIn.
To sum up this first exciting stage here are some of the insights of various participants from data holders, to clinicians and patients.

The European Health Data & Evidence Network (EHDEN) has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No 806968. The JU receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
OHDSI is a multi-stakeholder, interdisciplinary collaborative to bring out the value of health data through large-scale analytics. All solutions are open source. OHDSI has established an international network of researchers and observational health databases with a central coordinating centre housed at Columbia University.







