PIONEER Prostate Cancer Core Outcome Sets
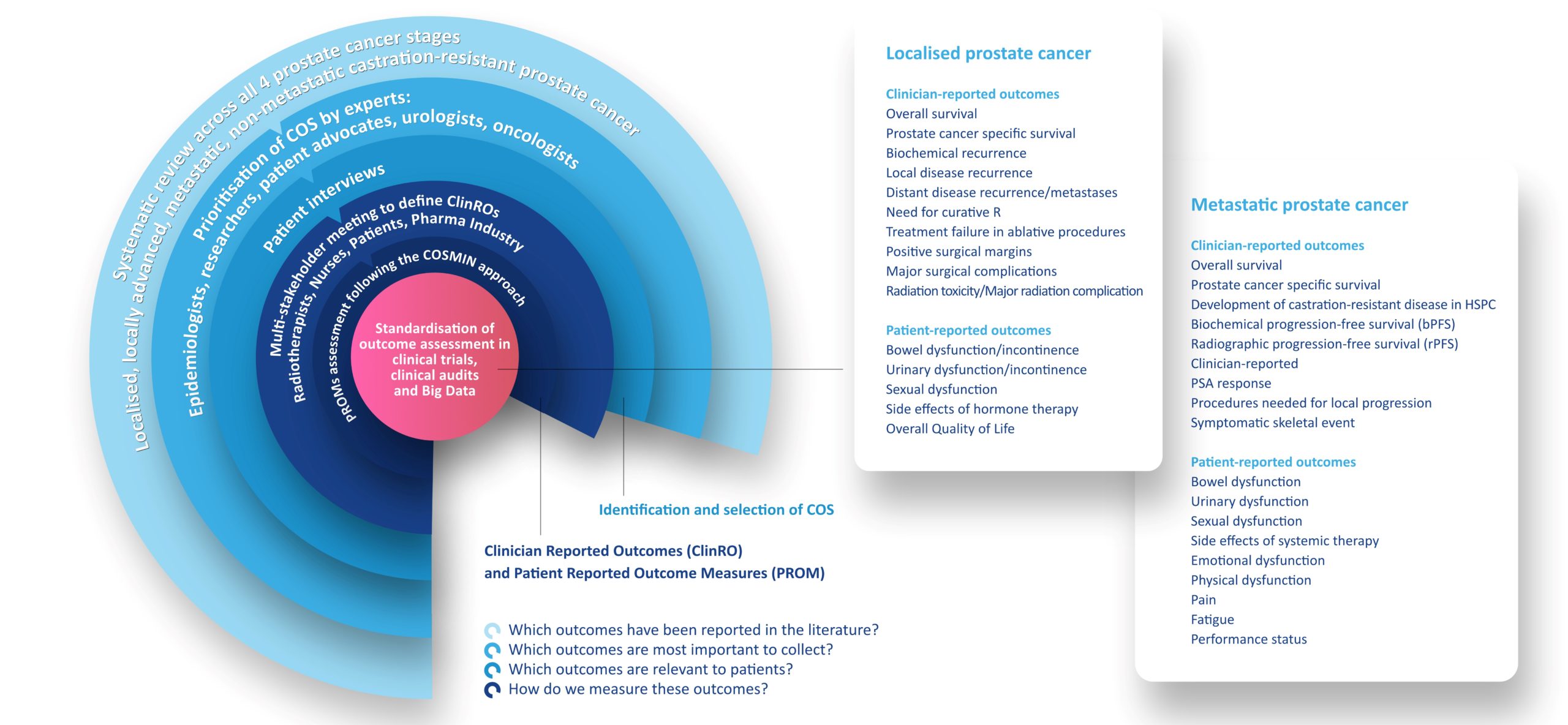
The PIONEER Core Outcome Sets (COSs) for Localised, Locally Advanced, Metastatic, and Non-metastatic Castration-resistant Prostate Cancer are the result of a methodological rigorous systematic review and consensus finding process with a diverse range of prostate cancer stakeholders including clinicians, nurses, researchers, industry partners and most importantly patients.
The PIONEER Prostate Cancer COSs have also been endorsed by the European Association of Urology, Europe’s leading medical authority on urological practice, research, and education. A reflection on the importance of COS for the advancement of clinical practice.
The PIONEER Prostate Cancer COSs are a continually evolving resource. They represent a minimum set of outcomes, which can be amended with defendable justification, depending on clinical setting and changes in the treatment landscape. Therefore, we would highly encourage everybody to start measuring these outcomes to improve treatment and care for prostate cancer patients.
Consensus Definitions PIONEER Core Outcome Sets
Patient Reported Outcome Measures
Prostate cancer is characterised by a relatively long natural history, and a substantial proportion of patients die from causes other than the disease itself. Therefore, assessment and monitoring of treatment-related side effects and of health-related quality of life (HRQoL) play a major role in the management of PCa patients.
There is increasing awareness of the importance of measuring what were previously regarded as softer outcomes, namely, side effects and HRQoL, using patient reported outcome measures (PROMs). This is crucial when considering that cancer patients may have the possibility of trading HRQoL for length of life.
PIONEER has systematically reviewed both clinician reported outcomes and the psychometric properties of Patient Reported Outcome Measures (PROMs) used in localised, locally advanced, and metastatic prostate cancer. Based on the results of the reviews and subsequent consensus processes, PIONEER recommended minimum sets of clinician and patient reported outcomes, provided definitions or each, and recommended PROMs, for use in routine practice and research settings
PIONEER Disease Understanding Work Package
Listen to Dr. Steven MacLennan and Prof. Mieke Van Hemelrijck explain what core outcome sets are and why they are important. Followed by Dr. Elena Sisca and Dr. Monica Ratti discussing patient reported outcome measures and their impact on clinical research and patient care.