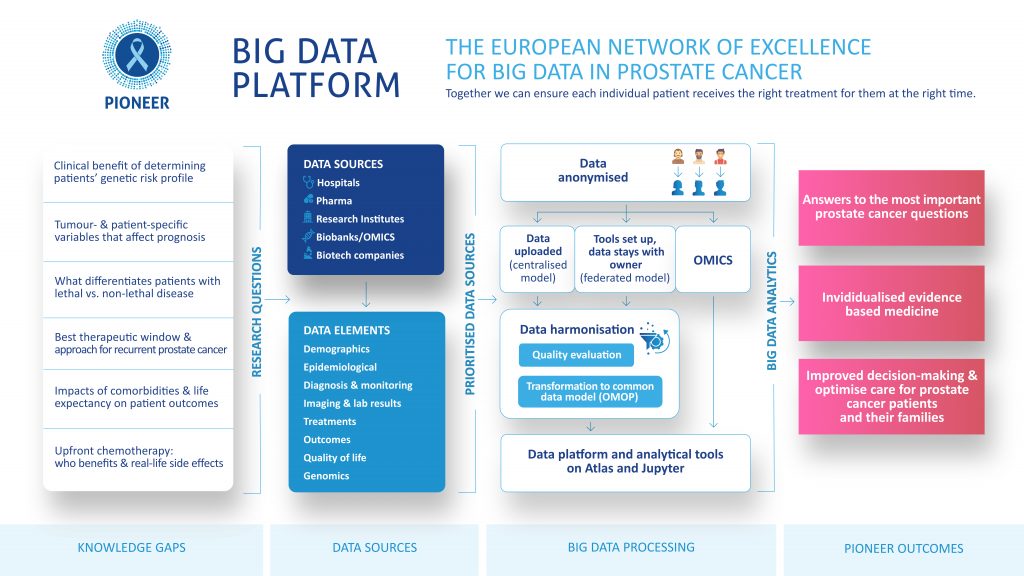
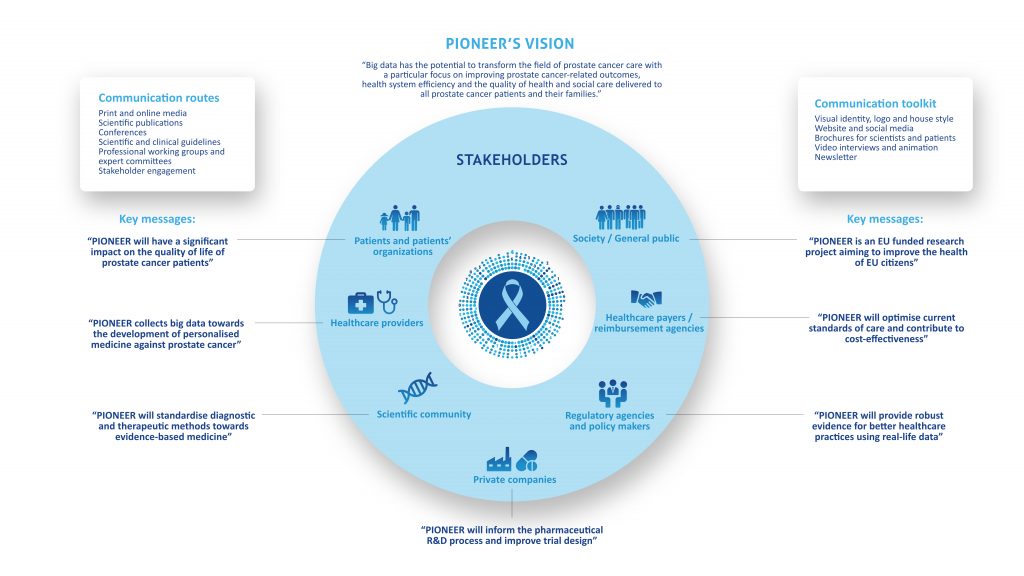
Launched in May 2018, PIONEER has a 5 year deadline in which to accomplish its objective of gathering, harmonising, analysing and combining broad data-sets on the diagnosis and treatment of Prostate Cancer into a comprehensive big data platform. To achieve its goal PIONEER has developed 8 individual work packages: project management and coordination (WP 1), 4 core research themes (WP 2-5) and 3 cross-cutting support themes (WP 6-8). For a detailed overview of each work package’s objectives and achievements to boxes below. WP1 will provide an effective project management programme and governance structure to ensure that PIONEER’s objectives are successfully fulfilled within the lifetime of the project. WP1 will facilitate collaboration between the consortium’s partners whilst ensuring all IMI2 requirements are respected. Objectives: Partners: European Urology Association, ASSOCIATION EISBM, University of Lund, Universita Vita-Salute San Raffaele, Bayer, Sanofi, Astellas Pharma and ttopstart. Download the PIONEER Big Data infographic WP2 will develop standardised definitions of prioritised prostate cancer outcomes and diagnostic and prognostic factors across the different stages of prostate care, whilst taking into account existing heterogeneity in data collection and the disease itself. WP2 aims to directly inform guideline development and clinical practice by standardising definitions and terminology regarding diagnosis, prognosis and outcomes for prostate cancer. The most important outcomes for different stakeholders will be prioritised and defined and a core outcome set will be developed for different stages of the disease. For each of the outcomes identified as core, the most appropriate and feasible outcome measurement instrument will be recommended. Consensus will also be sought for diagnostic tests and parameters, and prognostic factors. WP2 will feed into WP3, 4 and 5, whilst the results from WP4/WP5 will feedback into WP2. Objectives: Key achievements to date: Partners: European Association of Urology, King’s College London, Universita Vita-Salute San Raffaele, International Consortium for Health Outcomes Measurement, University of Aberdeen, European Organisation for Research and Treatment of Cancer, Bayer, Sanofi, Janssen Pharmaceutical, Orion Corporation and Astellas Pharma. WP3 will address the challenges related to identifying and prioritising potential data sources; negotiating appropriate data access agreements; homogenisation of data and the establishment of data management plans to support the long-term sustainability goals of PIONEER. To achieve its objectives WP3 will work closely with WP4 and WP8. Objectives: Key achievements to date: Partners: Erasmus Universitair Medisch Centrum Rotterdam, The Hyve B.V., Bayer, University of Lund, University of Tampere, Technische Universität Dresden, Fraunhofer IZI, Goeteborgs Universitet, Radboud University Medical Center, European Organisation for Research and Treatment of Cancer, Universitaetsklinikum Hamburg-Eppendorf, Varian Medical Systems, Astellas Pharma and Covance. The main aim of WP4 is to deliver a data repository and analytics platform that is secure, sustainable and meets PIONEER’s objective to improve prostate cancer outcomes by identifying the relevant outcome measures from epidemiological, clinical, economic and, patient-reported outcome data. To achieve its aim WP4 will work closely with WP3 and WP5. Objectives: Key achievements to date: Partners: The Hyve B.V., ASSOCIATION EISBM, Varian Medical Systems, Imperial College of Science Technology and Medicine, Bayer, Radboud University Medical Center, Astellas Pharma and Orion Corporation. The main aim of WP5 is to carry out analyses of the data sources identified and collated by WP3 and WP4 based on the core outcome sets for localised, locally advanced, and advanced-metastatic prostate cancer identified by WP2 in order to answer, or provide opportunities to answer, the main questions related to prostate cancer. Objectives: Key achievements to date: Partners: ASSOCIATION EISBM, Erasmus Universitair Medisch Centrum Rotterdam, Universitaetsklinikum Hamburg-Eppendorf, European Organisation for Research and Treatment of Cancer, Imperial College of Science Technology and Medicine, Weizmann Institute of Science, Fraunhofer IZI, Swedish Institute for Health Economics, Astellas Pharma, SAS Institute, Orion Corporation and Bayer. The main aim of WP6 is to identify and address evidence gaps which can delay decision-making by regulatory agencies, health technology assessment (HTA) bodies and payers, which ultimately compromises timely patient access to innovative treatments for prostate cancer. WP6 will build upon previously gained experience in other EU-funded projects and international initiatives and will provide advice and support to work packages 2, 3, 4 and 5 to ensure that the HTA-, patient-, regulator-, and payer-relevant perspectives are reflected. Objectives: Key achievements to date: Partners: European Alliance for Personalised Medicine, Swedish Institute for Health Economics, Janssen Pharmaceutical, Bayer and Sanofi. WP7 will run in parallel with the other packages during the entire lifecycle of the project and focuses on dissemination and communication of the project’s aims, objectives and results. Its key aim is the widest possible dissemination of the project’s results to both the research community and all other interested/identified stakeholders. IMI supports collaborative research projects and builds networks of industrial and academic experts to boost pharmaceutical innovation in Europe. WP7 aims to establish links with this community, facilitating effective communication with the other BD4BO projects to ensure that PIONEER’s objectives are well known. Objectives: Key achievements to date: Partners: European Association of Urology, European Cancer Patient Coalition, ecancer Global Foundation, Bayer and Sanofi. Download the PIONEER communication graphic here The main aim of WP8 is to develop a legal and ethical framework to provide guidance on addressing patient confidentiality and data ownership concerns, and establish sustainable governance for the data infrastructure. The timing of PIONEER will coincide with the coming into force of the General Data Protection Regulation (GDPR) in May 2018, the most significant change in data privacy law in over 20 years. WP8 will closely track how EU members States may derogate from GDPR and how regulatory or professional bodies provide specific guidance on application to patient data and big data. WP8 collaborate with, and provide guidance to other WPs (3, 4 and 5 in particular). WP8 will develop and share best practice as it emerges throughout the term of the lifetime of PIONEER and report regularly on progress. Objectives: Key achievements to date: Partners: European Organisation for Research and Treatment of Cancer, Pinsent Masons LLP, ASSOCIATION EISBM, Bayer, Astellas Pharma and Orion Corporation.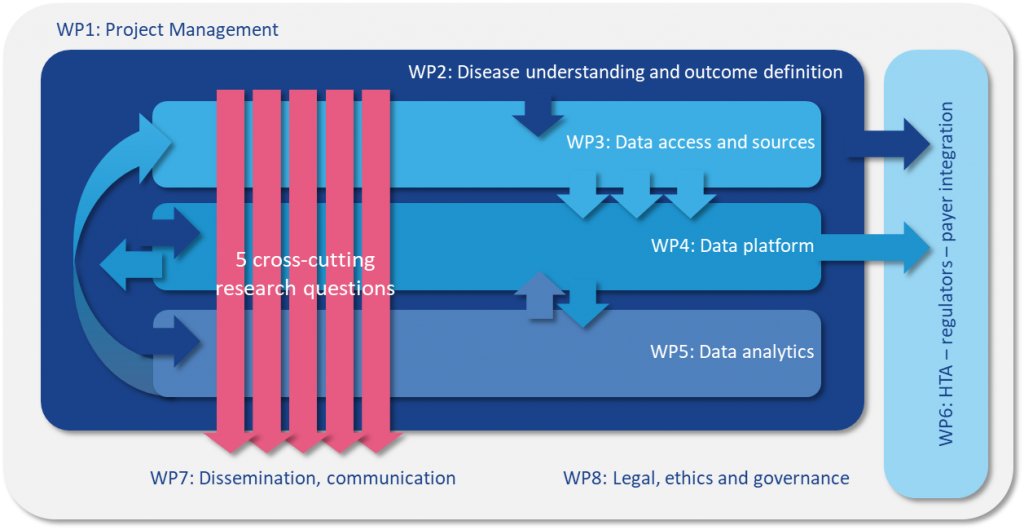
Work package 1 project management & administration
Work package 2 disease understanding & outcome definitions
Work package 3 data access & sources
Work package 4 data platform
Work package 5 data analytics
Work package 6 HTA regulator-payer integration
Work package 7 dissemination & communication
Work package 8 legal ethics & governance
Contact us here



