
Big Data for Better Outcomes
The Big Data For Better Outcomes Programme (BD4BO) is an umbrella programme of the Innovative Medicines Initiative 2 (IMI2). IMI2 is a collaboration between the European Union and European Federation of Pharmaceutical Industries and Associations (EFPIA) and is the world’s largest public-private partnership (PPP) in the life sciences. EFPIA companies and associated partners do not receive any EU funding, but contribute to the projects “in kind”, for example by contributing their researchers’ time or providing access to research facilities or resources.
The aim of the Big Data For Better Outcomes Programme (BD4BO) is to improve health outcomes and healthcare systems in Europe by maximising the potential of Big Data.
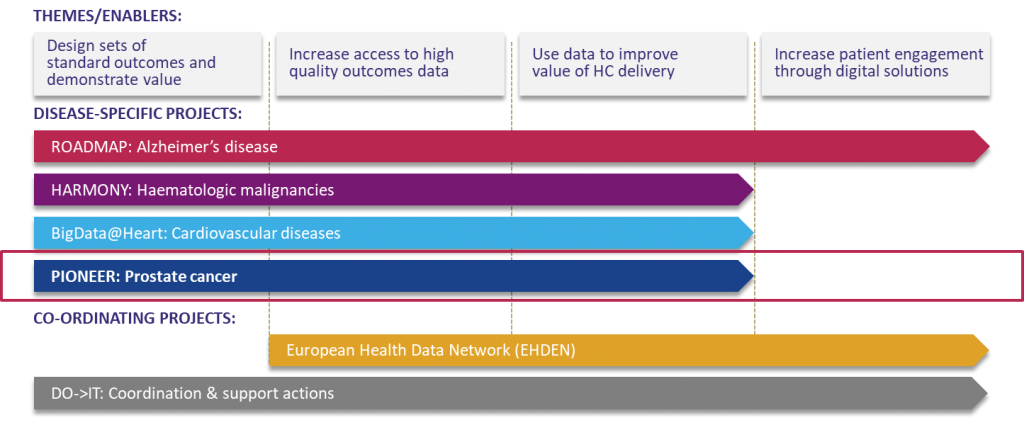
The BD4BO programme comprises several disease-specific projects focusing on Alzheimer’s disease (ROADMAP), hematologic malignancies (HARMONY), cardiovascular diseases (BigData@Heart), and prostate cancer (PIONEER). These disease-specific projects are supported by the Coordination and Support Action as an overarching coordination structure, and the European Health Data Network whose objective is to establish a federated network of relevant and high-quality data sources across Europe.
Fundamentally, Big Data has the potential to have a transformative effect across a board spectrum of areas including healthcare systems, patient stratification and disease treatment, trial and product design, and most importantly, patient use of medicines. However, the potential of Big Data will only be unlocked when healthcare systems move beyond the mere collection of large amounts of data. To achieve its potential previously separated data sets must be linked and analysed using suitable Big Data analytics. This approach will offer all stakeholders novel ways to accelerate research and to identify the right treatment for individual patients (personalised medicine). Access to large combined data sets that present a more comprehensive picture of patients allows patient-reported outcomes to be measured more accurately. If they are of high enough quality and/or supported by other findings such as clinical trial data they can support decision-makers in shaping patient focused healthcare systems.
The Innovative Medicines Initiative
The IMI mission is to improve health by speeding up the development of, and patient access to, innovative medicines, particularly in areas where there is an unmet medical or social need. They do this by facilitating collaboration between the key players involved in health research, including universities, research centres, the pharmaceutical and other industries, small and medium-sized enterprises (SMEs), patient organisations, and medicines regulators. The IMI is the world’s biggest public-private partnership in the life sciences. It is a partnership between the European Union represented by the European Commission and the European pharmaceutical industry represented by the European Federation of Pharmaceutical Industries and Associations (EFPIA). Through the IMI2 programme, they have a €3.3 billion budget for the period 2014-2020.
The goal of the IMI is to develop next generation vaccines, medicines and treatments. IMI projects will provide Europeans with more efficient and effective medicines and treatments. Whilst greater coordination across industry sectors will result in more reliable and faster clinical trials, and better regulation.
For more information, go to the IMI and BD4BO website.



